
.png)

.png)
☢PROMETHIUM (Pm)//
Thy Godlike crime was to be kind, / To render with thy precepts less./ The sum of human wretchedness, / And strengthen Man with his own mind.
Promethium is element number sixty-one on the periodic table. Promethium is a lanthanide, making it a metal and is the only element within the lanthanide group that is a synthetic element. Promethium uses the symbol Pm. Since it’s element sixty-one, that means it has sixty-one electrons and protons, with eighty-four neutrons. Promethium is the only element within the lanthanide group that is radioactive, as well. It is highly unstable, with no non-radioactive isotopes at all.
Promethium, at room temperature, is a silvery-white solid. Being a rare earth metal, it has a very noticeable metallic luster on it. Because it's a part of the lanthanide family, it is malleable and ductile, as well as dense, with a density of 7.26g/cm^3. Which probably means nothing to you, so as a comparison, it is a bit less dense than iron and more comparable to that of tin.

It has both a melting point and a boiling point temperature, with the melting point being 1,042 degrees Celsius and the boiling point being 3,000 degrees Celsius. It is conductive, though not as conductive as something like copper. Like a lot lower actually, it has a low thermal and electric conductivity.
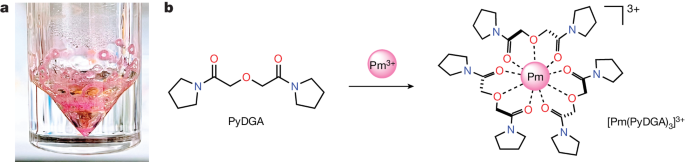
Because not many research studies have been conducted to promethium, given that it's an extremely rare element, its reactions can only be predicted. In cold water, promethium may form promethium hydroxide slowly, and in hot water it may form hydrogen gas. Promethium is known to be highly reactive with oxygen, forming promethium oxide. Promethium oxide is the most common form of promethium, and is a pale pink compound. Promethium is reactive enough to air that it poses a significant explosion risk in its powder form. It is very flammable in powder form. Promethium also reacts with acids, notable compounds being Promethium chloride and Promethium fluoride (these salts also tending to be heavily luminescent!). Promethium is heavily radioactive, making it highly toxic for humans.

Promethium was originally theorized to have existed in 1902 when it was noted that there was an empty space between neodymium (element 60) and samarium (element 62). This was confirmed by Henry Moseley, a physicist who in 1914 found that element 61 was indeed missing from the periodic table. It wouldn't be until 1938 that an experiment unrelated to promethium would be the first evidence of it existing. However, it wasn't conclusive enough to prove its existence until it was produced fully in 1945 at Oak Ridge National Laboratory, after they separated and analyzed broken down results of uranium. It was discovered by Jacob A. Marinsky, Lawrence E. Glendenin and Charles D. Coryell, but they didn't announce this discovery until after the end of World War II, and after they were done their research from the war. It was announced in 1947. The etymology of the name "promethium" is derived from the Greek Titan Prometheus, who had stolen fire and given it to mankind.
Promethium-147, an isotope of promethium, is often used in atomic batteries. These batteries specifically are small (thumbtack-sized) and long-lasting. These batteries are often used for satellites and moon landers. Promethium is useful for this because, when it decays, it releases particles and energy that provide electricity, similar to a solar panel or cell. Historically, promethium has been used in luminous paints for that reason as well, as it glows when it decays. This was most notably used during the Apollo missions by NASA, where they used this paint to light up buttons and modules. Promethium is also useful in measuring thickness. This is used by measuring the amount of radiation from the promethium that passes through whatever is being measured.

Promethium doesn't exactly pose a significant risk of harm, given that its radiation is easily shielded by a thin layer of any given material. Promethium has been tested on a variety of animals and even humans. Within the body, it becomes localized within the bones and surrounding them, as well as some deposits in the gastrointestinal system. It doesn't pose a massive risk to the environment due to its practical non-existence within it, though improperly stored promethium can become dangerous for plants and wildlife given its radioactive nature. Little has been found about its effects on the human body, though when inhaled it is stored in the lungs without much gone.

Though it was initially theorized that there are "500-600 grams of promethium in the Earth's crust at any given time," searches for natural deposits of promethium have not yielded much results. Instead, natural promethium has been detected on a star within the Andromeda galaxy.
Promethium as a name was suggested by one of the discoverer's wives, as it as an element is reminiscent of "both the daring and possible misuse of the mankind intellect," a reference to how Prometheus disregarded Zeus and stole fire to enlighten mankind, sacrificing himself for humanity.
Promethium is not at all long lasting, the longest isotope known is Promethium-147, which only lasts for around 17-18 years. After that, it breaks down into elements 60 or 62, neodymium and samarium respectively. The shortest known isotope is Promethium-128, which was recorded to have lasted one second.
.png)
https://periodic-table.rsc.org/element/61/promethium
https://www.webelements.com/promethium/chemistry.html
https://www.frontiersin.org/journals/chemistry/articles/10.3389/fchem.2020.00588/full
https://periodictable.com/Elements/061/data.html
https://en.wikipedia.org/wiki/Promethium
